LATEST NEWS

UKAS ISO 17025 accredited Single Tube Method for Determining the Efficacy of Disinfectants against Candida albicans Biofilm
Perfectus Biomed Group are excited to announce that we have recently received UKAS 17025 accreditation for the extension to scope for the Single Tube Method to include Candida albicans as a test organism. We are industry leaders in biofilm testing methods, please contact...
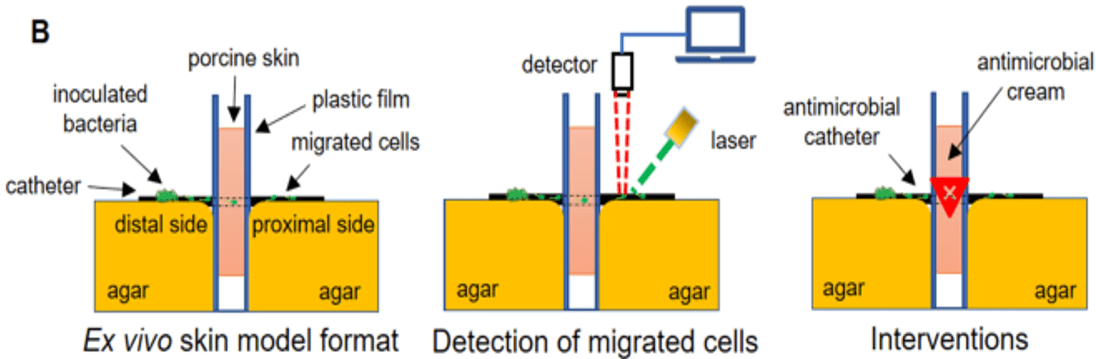
Perfectus Biomed work with the FDA to develop an ex vivo pig skin model of IV catheter related infection
Perfectus Biomed are experts in developing ‘fit for purpose’ experiments to mimic real life scenarios. In partnership with the FDA, Perfectus Biomed have developed and published in Nature, the first ex-vivo porcine skin-catheter model and accompanying test methods...
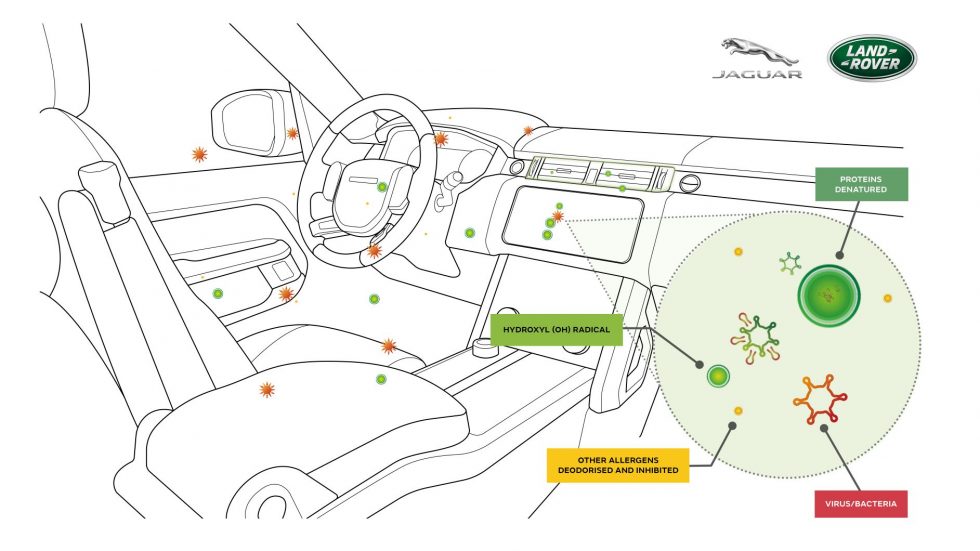
Perfectus Biomed works with Jaguar Land Rover to prove air purification technology inhibits viruses
Jaguar Land Rover’s future cabin air purification technology has been shown in laboratory tests to inhibit viruses and airborne bacteria by as much as 97 per cent.
Jaguar Land Rover partnered with Perfectus Biomed Ltd to perform the world-leading...

Alternatives to clinical studies from Perfectus Biomed
An estimated 80% of non-COVID-19 clinical trials were stopped or interrupted due to the global pandemic according to Aaron von Dorn writing in The Lancet in August 20201. What are your options when this happens?
Lockdown has highlighted the vulnerabilities in clinical...

And the winner of the Bionow Technical Service Award is…
We are very excited to announce that Perfectus Biomed are the winners of the 2020 Bionow Technical Service Award.
The night’s ‘virtual’ celebrations were hosted by Bionow, however we still indulged in the excuse to dress up and have a glass of fizz. A brilliant...
Collaboration in Isolation
Merging two companies is always a daunting task but doing it successfully, smoothly and across two continents in some of the strangest times in a generation is nothing short of remarkable. On the 2nd June that’s exactly what Perfectus Biomed and Extherid...
Perfectus Biomed elevate method testing beyond the ‘standard’
Perfectus Biomed are delighted to announce that they have once again expanded their range of UKAS accredited test methods. In addition to their accredited biofilm methods, the latest Extension to Scope to ISO 17025 sees a remarkable achievement to accredit an Ex-vivo Porcine Lung Tissue Model. Ordinarily ISO 17025 is applied to current standard methods, typically carried out in suspension or on abiotic surfaces. Accrediting reproducible and reliable tissue based models is pivotal in creating models that represent complex ‘real world’ conditions and therefore in developing safe and effective treatments and therapies.
In contrast EN 1276 is a straightforward, widely used biocide testing standard. Accrediting the ‘everyday’ standards and methods to ISO 17025 offers customers an extra level of confidence and affirms credibility.
NBIC Launch #BiofilmAware Campaign
Perfectus Biomed are excited to support The National Biofilms Innovation
Centre (NBIC) as they launch their biofilm awareness campaign, #BiofilmAware,
which will run over the next 12 months.
Biofilms are well known to a number of research...
Perfectus Biomed’s innovative response to COVID-19 secures funding
Perfectus Biomed are delighted to learn that our innovative response during the pandemic to develop rapid COVID-19 screening for businesses has been recognised and that we have secured funding from the Liverpool City Region Future Innovation Fund.
