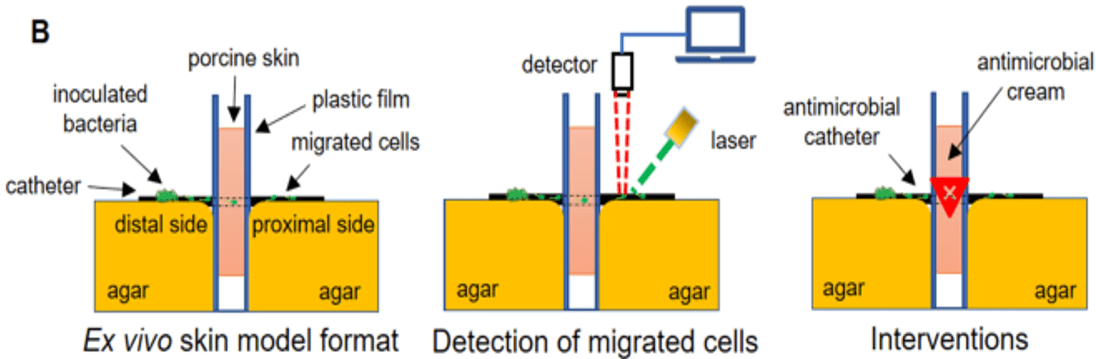
Indwelling medical devices, such as catheters, break the skin barrier that normally protects us from harmful bacteria, greatly increasing the risk of infection. Ex vivo tissue models replicating early stages of microbial contamination, ingress, and colonization leading to infection, provide valuable information at the preclinical stage in medical device development, bridging the gap in understanding between in vitro testing and clinical outcomes. Furthermore, models like this reduce the need for animal testing and support claims towards FDA approval of medical devices and antimicrobials.
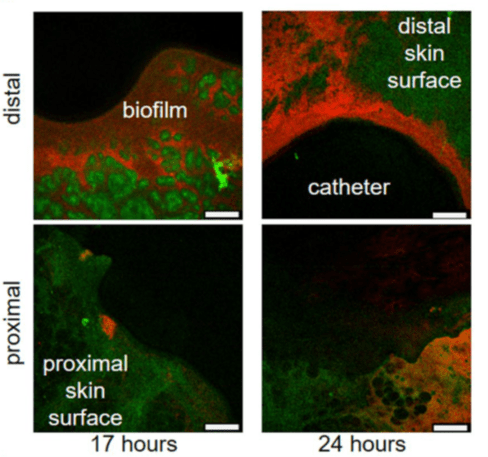
Skin surface autofluorescence is green, RP437/pRSH103 E. coli is red, and the black region is insertion site of catheter. Images for each timepoint were taken of sections around the catheter, as seen by the curvature where the biopsy punch was made. Distal side is the epidermis and proximal side is mostly hypodermis and associated fat cells.
The importance of including tissue in preclinical models is highlighted by the difference in results obtained from in vitro versus the ex vivo assays. The tissue models replicate several factors that the in vitro testing cannot, for example, challenges posed by biofilms (versus planktonic bacteria) which are more recalcitrant to eradicate on ex vivo tissue than on plastic plates or flow cells. Tissue can also absorb antimicrobials resulting in suboptimal inhibitory concentrations and reduced bactericidal efficiency compared to expected results from in vitro studies.
Perfectus Biomed have pioneered the development of UKAS accredited ex vivo models, including an ex vivo porcine lung tissue model which is especially useful to model chronic infections and antimicrobial interventions. We have extensive experience of working with tissue models, which are highly customizable and can be easily tailored to fit your needs, as well as imaging and visualization techniques to demonstrable outcomes.
Speak to us today about our customizable ex vivo tissue models by emailing info@perfectusbiomed.com or call 01925 737237