LATEST NEWS

Edge Hill University’s Alumni of the Year awarded to George Gray
George Gray, our Deputy Quality Manager, recently received his Alumni of the year trophy from Edge Hill University. The award was earned through George's work with Edge Hill's Biology department to enhance the knowledge of the industry, thus boosting employability by...

World Antimicrobial Awareness Week
Antibiotic resistance is one of the biggest threats to global health. A worldwide action plan issued by the World Health Organisation (WHO) aims to improve awareness of antimicrobial resistance, support R&D in the area and ensure sustainable investment to reduce...

EN 14476: Chemical disinfectants and antiseptics standard
Perfectus Biomed Group, Magnitude Biosciences, and University of Kent Project Funded by National Biofilms Innovation Centre for Tackling and Exploiting Biofilms
A joint project for Perfectus Biomed Group, Magnitude Biosciences, and the University of Kent aims to provide an assay to aid research on infections caused by Pseudomonas aeruginosa, delivering a solution to accelerate wound care research and reduce the reliance on...
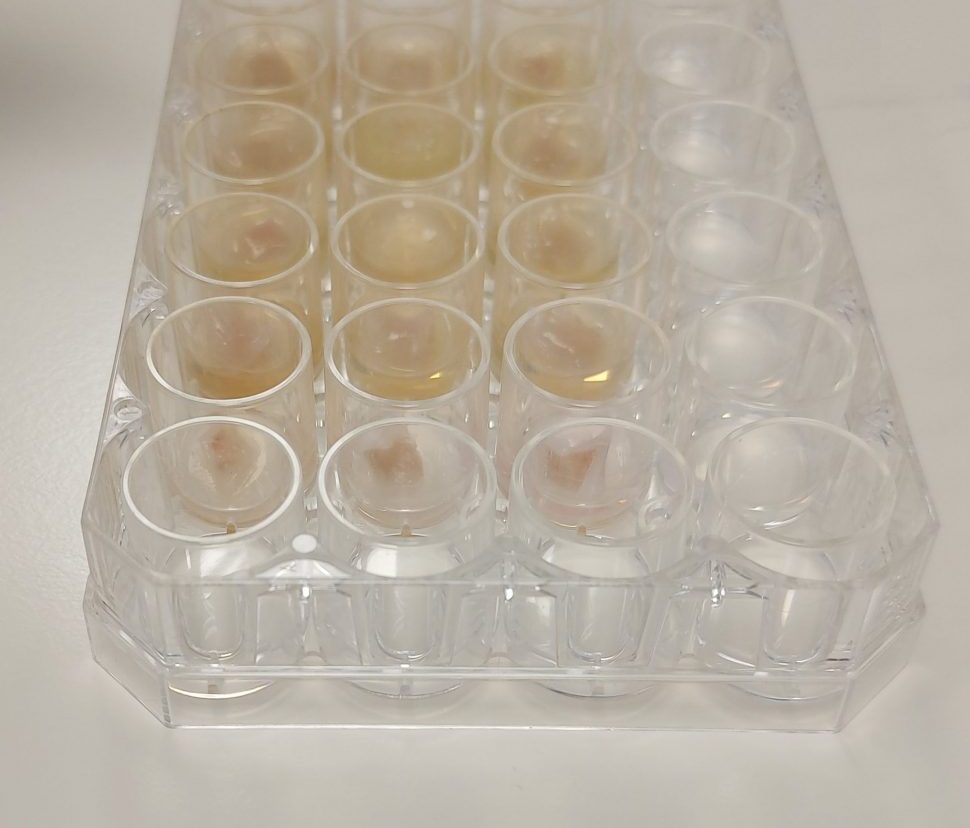
Accredited ex vivo Lung Model Biofilm Testing
Biofilm Awareness Week
Perfectus Biomed Group are excited to support The National Biofilms Innovation Centre (NBIC) #BiofilmWeek, highlighting the research taking place to prevent, detect, manage, and engineer biofilms. Perfectus Biomed’s mission is to harness...
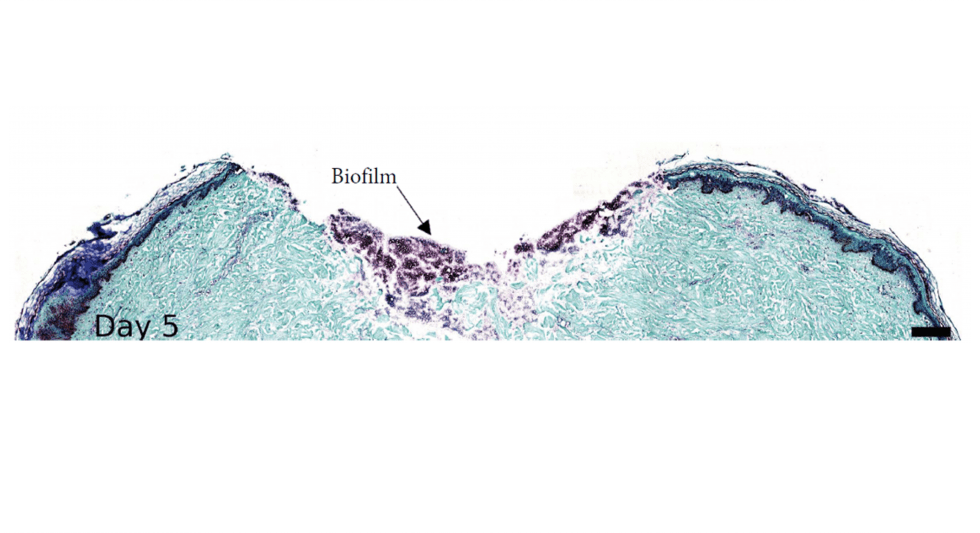
A wounded ex vivo human skin model to study microbial biofilm development and anti-biofilm therapeutics.
Perfectus Biomed Group have an actively innovative culture, and we strive to practice science that improves lives. Our unique specialism in biofilms, and our experience in customisable tissue models built the base for this particularly interesting study.
Biofilm forming...

Cell Line Quality Control and Authentication – Are your cells what you think they are?
Do you always know what cell line you are working with? I am sure most of you reading this right now are nodding, confident in your cell line quality control, however there is a widely unknown reproducibility crisis that is threatening viral and mycobacterium contamination...
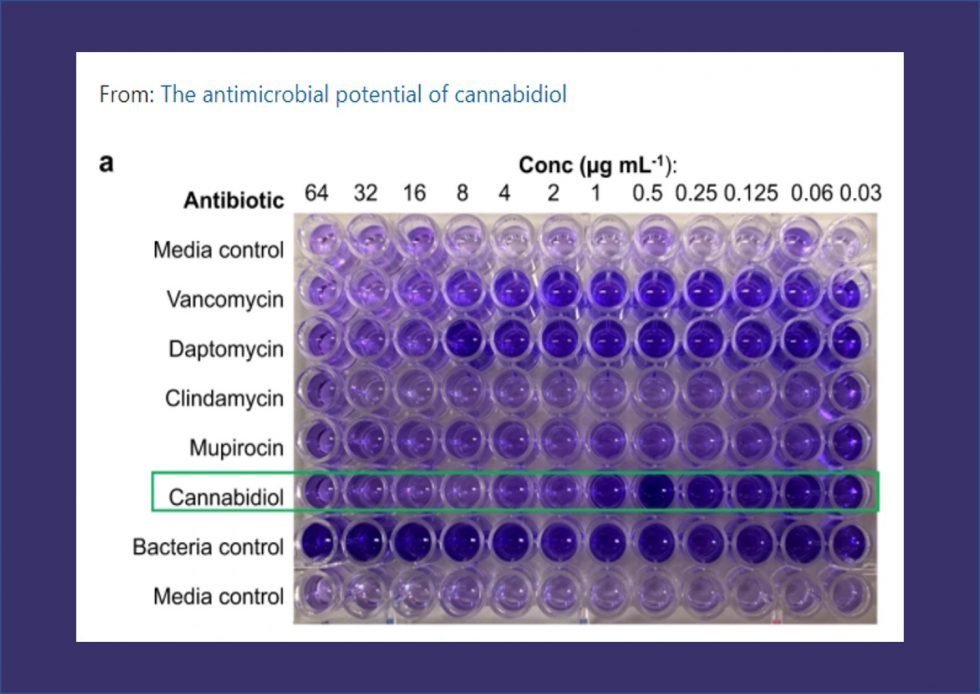
Perfectus Biomed Group Publish Paper – The Antimicrobial Potential of Cannabidiol
Perfectus Biomed Group are leading the market with accredited biofilm test methods, and research & development. Our team in the US collaborated on a paper published in Communications Biology, that explored the antimicrobial potential of cannabidiol. The...
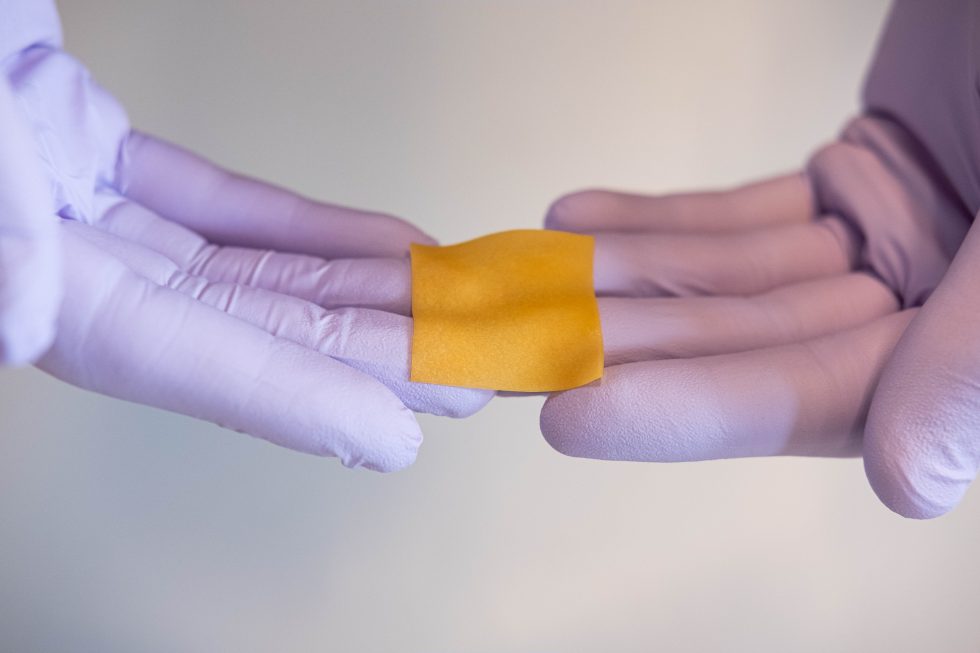
A Sweet Collaboration – Perfectus Biomed and SweetBio publish joint paper
Perfectus Biomed Group and SweetBio Inc. have published a joint paper in Biomedical and Translational Science exploring the activity and efficiency of a novel bioengineered wound product (BWP) and its potential to help combat infection. Perfectus Biomed regularly...
