What is Monkeypox?
On the 23rd July 2022, the World Health Organisation declared the spread of monkeypox as a public health emergency of international concern. Countries in Europe have recorded high cases, but there have also been reports in the US, Canada, Australia, Nigeria, Israel, Brazil, and Mexico, among others.
Human monkeypox was first identified in humans and recognised as a human disease in the 1970’s,1 outbreaks have continued to occur globally with the most recent in May 2022. Transmission most commonly occurs from close skin-to skin contact with an infected person; however, the virus can also survive on linens and clothing (such as bedding and towels) used by an infected person. Symptoms include fever, headaches and backpain. Once the fever breaks a rash can develop, this can be extremely itchy or painful and may lead to skin lesions. The infection usually clears up on its own and lasts between 14 and 21 days.2 The CDC’s recommended measures for infection control include – patient isolation, use of PPE and standard cleaning and disinfectant procedures using an EPA registered hospital grade biocide.
Perfectus support antiviral product development
Where the organism of interest is significantly pathogenic, it is commonplace to test product efficacy against a viral surrogate. A viral surrogate is an organism that is similar to the pathogen but is also a) safer to handle than the pathogen, b) easier to grow to an appropriate test titre and to test in vitro than the pathogen, c) presents a treatment challenge than is at least as challenging as the pathogen. The antiviral efficacy of a formulation against the Monkeypox virus can be demonstrated by testing against an enveloped virus that is similar in structure to Monkeypox. Vaccinia is the enveloped virus used in the EN 14476 and EN 16777 standard tests to evaluate viricidal activity and proven activity against vaccinia allows for antiviral claims against all enveloped viruses. . Perfectus Biomed perform standard, adapted standard and customised methods to test anti-viral product efficiency.
A relation of smallpox
Monkeypox belongs to a subset of the Poxviridae family called Orthopoxvirus. Features of the Poxviridae family include duel stranded DNA, linear genome, and they can typically be visualised with light microscopy. (Orthopoxvirus has four members of close relations. Variola (smallpox), Vaccinia, Cowpox, and Monkeypox (Figure 1).
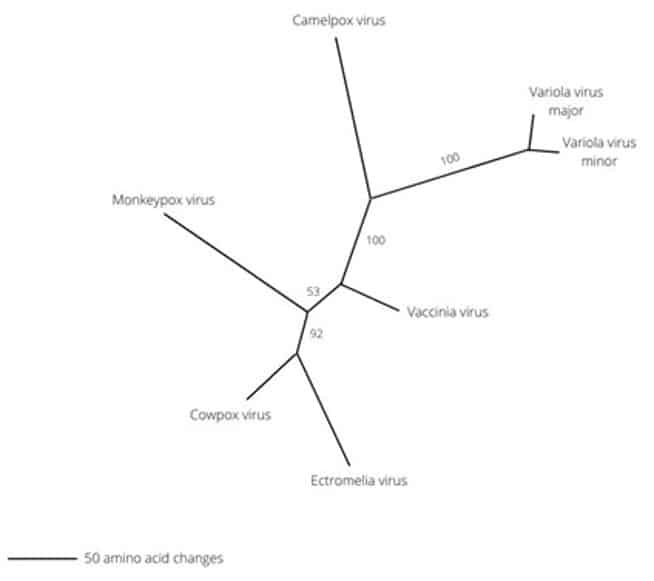
Figure 1. Evolutionary tree of the genus Orthopoxvirus.3
Generally, the viral structure of this organism is large and rectangular in shape. All pox viruses have a central core, which is biconcave, containing DNA and viral proteins. The virus has one or two envelopes with associated glycoproteins.
Industry response to the 2022 Monkeypox outbreak
To safeguard public health and reduce the risk of human-to-human transmission, infection control precautions have been recommended. This includes safe transportation of samples, suitable PPE for healthcare staff handling samples and an emphasis on hand hygiene (soap and water or use of an alcohol-based hand sanitizer) and disinfectant use to limit further onward spread. In response to this recent virus emergence, regulatory bodies have reviewed guidelines to support manufacturers. ln a recent news post, the TGA (Australian Government’s Department of Health and Aged Care, Therapeutic Goods Administration) advised that Vaccinia virus is the recommended surrogate virus for manufacturers wishing to make label claims of efficacy against Monkeypox for products that are either hard surface disinfectants or disinfectants that are medical devices.4
The EPA (United States Environmental Protection Agency) recently activated its Emerging Viral Pathogens (EVP) guidance, this is a process which allows disinfectant manufacturers to publicly communicate efficacy claims against the monkeypox virus. If an EVP claim is approved for a given disinfectant, specific off-label claims can be made in the event of an outbreak and the subsequent activation of the EVP policy. A statement indicating efficacy against virus similar to monkeypox can be made in product technical literature distributed to clinicians, marketing claims and consumer information services. The EPA have also published a “List Q” of all registered disinfectant products currently approved for use against EVP.5
Our dedicated team can support clients to evaluate and assess the viricidal efficacy of their products, contact us to discuss your latest project.
References
- https://www.who.int/news-room/fact-sheets/detail/monkeypox
- https://www.nhs.uk/conditions/monkeypox/
- https://www.semanticscholar.org/paper/Looking-back-at-smallpox.-Bray-Buller/2c8126a471e63f721f66f02ad4aedf0c3ff6ea75/figure/0
- https://www.tga.gov.au/behind-news/surrogate-viruses-use-disinfectant-efficacy-tests-justify-claims-against-monkeypox
- https://www.epa.gov/pesticide-registration/disinfectants-emerging-viral-pathogens-evps-list-q