BS EN 14476
VIRAL TESTING SPECIALISTS
BS EN 14476 2013+A2: 2019 – Quantitative suspension test for the evaluation of virucidal activity in the medical area.
Test Conditions
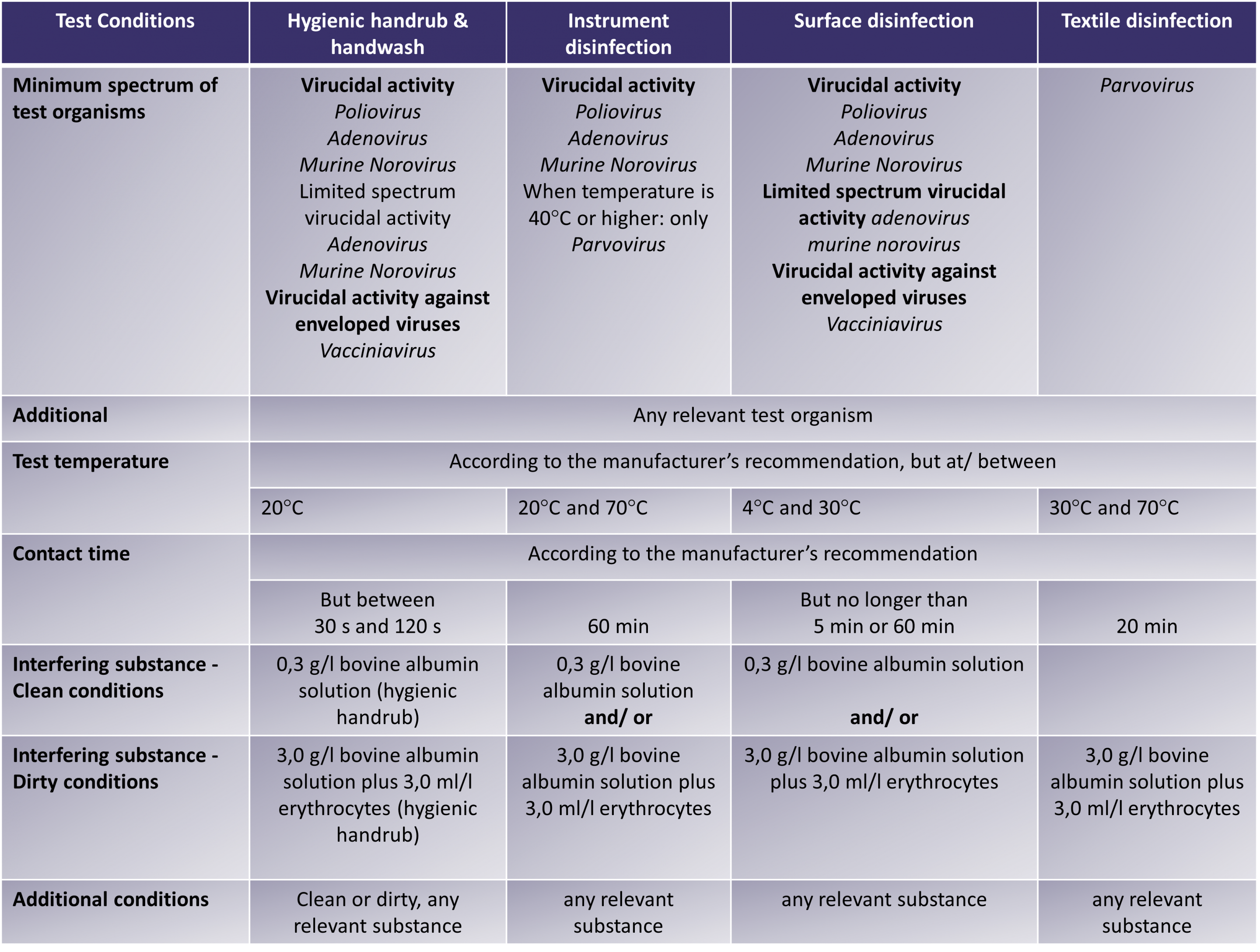
The below table outlines the minimum requirements for the EN 14476 test:
This standard specifies the minimum requirements for viricidal activity of chemical disinfectant and antiseptic products that form a homogenous physically stable preparation when diluted with hard water.
Common products that require this standard
- Hygienic handrub/ handwash
- Instrument disinfection
- Surface disinfection – wiping/spraying/flooding
- Textile disinfection
The EN 14476 standard applies to areas and situations where disinfection is medically indicated. Such as hospitals, community medical facilities, dental institutions, school clinics, nurseries and nursing homes. For a hand product to be fully virucidal or acknowledged as capable of inactivating all enveloped and non-enveloped viruses, it must be effective against adenovirus, norovirus and poliovirus. Instrument and surface disinfectants intended for the medical area however, must pass the test against all three non-enveloped viruses.
The sample is added to a test suspension of viruses in a solution of an interfering substance. At the end of the contact time an aliquot is taken, the viricidal action in this portion is suppressed for the duration of the exposure time at the temperature specified by the manufacturer.
At the end of the exposure time, samples are retrieved and the activity of the test product is neutralised by dilution in ice-cold test medium. Serial dilutions are performed, and the dilutions are examined for viral infectivity.