BS EN 16777
VIRAL TESTING SPECIALISTS
BS EN 16777: 2018 – Chemical disinfectants and antiseptics – Quantitative non-porous surface test without mechanical action for the evaluation of virucidal activity of chemical disinfectants used in the medical area
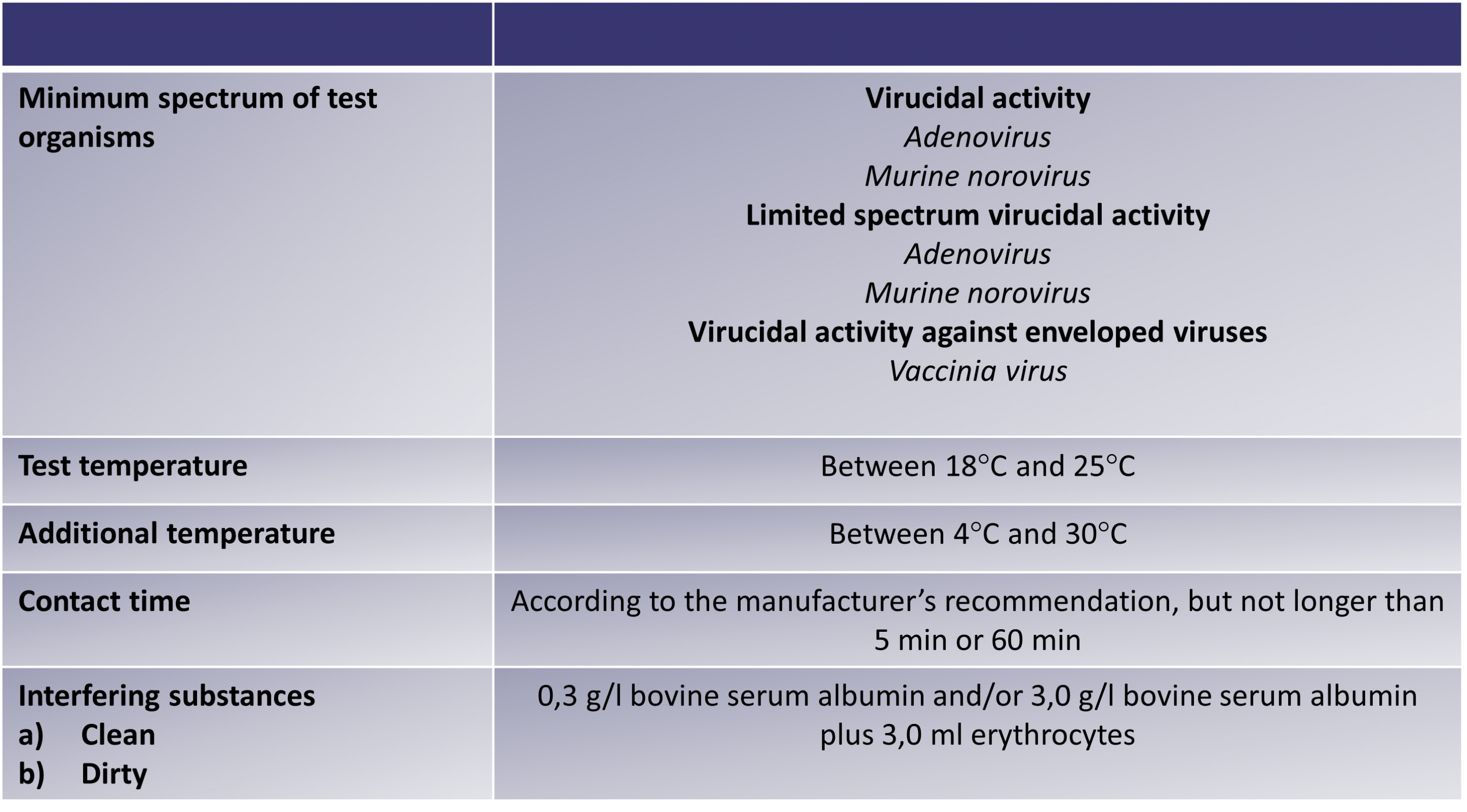
Test Conditions
The below table outlines the minimum requirements for the EN 16777 test:
This standard describes a surface test method for establishing whether a product proposed as a disinfectant has or does not have virucidal activity on non-porous surfaces.
This standard applies to areas/situations where disinfection is medically indicated, such as the following:
- in hospitals
- community medical facilities
- dental institutions
- nursing homes
clinics in schools
A sample of the product is added to a test suspension of viruses in a solution of an interfering substance, inoculated onto a test surface and dried. The surface is then submerged into a neutralizer solution to stop the active ingredients from working beyond the contact time. A sample of the neutralizer is acquired, plated and incubated.