Cell Banking and Cell Culture Services
Cell Banking you can trust.
Your cell banks, maintained at an exceptional quality, available at your request.
In-vitro cell cultures are a vital resource in scientific research & development. Their importance has increased in recent years due to an on-going focus to reduce animal testing and due to their usage in highly reproducible, high throughput, In vitro assays. Cell cultures are an excellent scientific tool however, maintaining a cell line in continuous or extended culture can increase the risk of contamination, research errors and inappropriate and costly research conclusions.
In collaboration with Cheshire based, life sciences start-up, Cryoniss we offer a complete cell banking package, from standard and customised cell banking to standalone quality control testing of cell lines/banks and Cryogenic storage. Outsourcing your cell banking and logistical needs to an expert, GLP complaint CRO ensures peace of mind. When you work with us, you get an end-to-end cell banking package, freeing up your in-house team to concentrate on their research.
Why outsource you cell bank?
As many as 31% of cell cultures used by laboratories are estimated to be contaminated with Mycoplasma and/or a second cell line. Working with an expert service provider to generate high quality cell banks helps to ensure the accuracy of your data and to de-risk research decision milestones within your organisation1.
Bank with us and receive:
- The cell banks you need when you need them.
- Peace of mind that all your cell banks are quality controlled in accordance with ATCC ASN-0002-2011
- Hassle free Global transportation of samples and long term cryogenic storage.
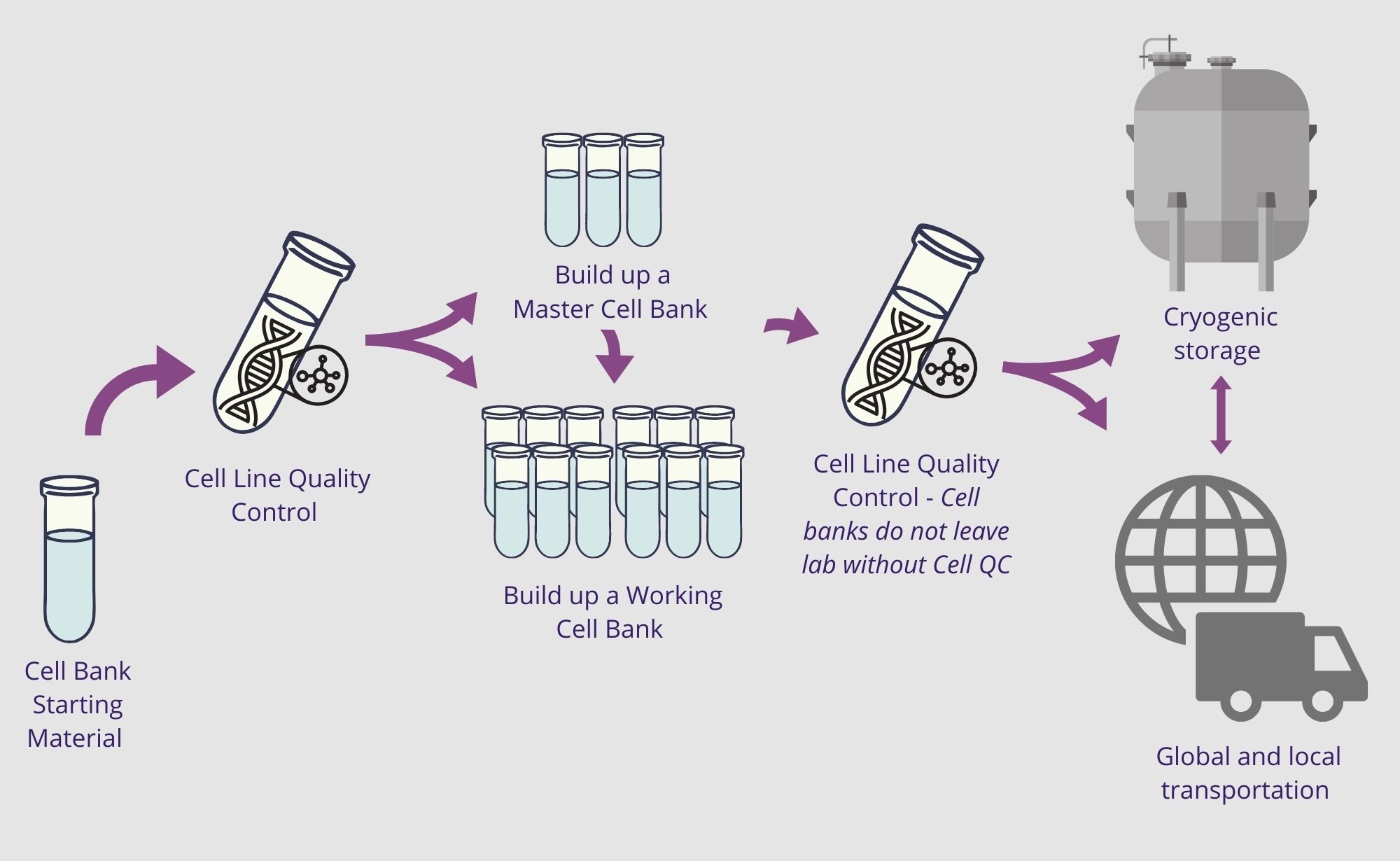
Cell banking support at all stages
We will work with you to coordinate a support option that meet your specifications and ensure the integrity of your precious cultures. Explore the options available in the below graphic.
Included in ATCC ASN-0002-2021 cell line quality control
- STR profiling
- Cell line authentication
- Mycoplasma testing
- Sterility testing
- Pathogen detection
- Post-thaw growth curve and visual cell sterility check
- Morphology check and accompanying image at 70% confluency