Do you always know what cell line you are working with? I am sure most of you reading this right now are nodding, confident in your cell line quality control, however there is a widely unknown reproducibility crisis that is threatening viral and mycobacterium contamination in your cell lines.
Scientists often attempt to reproduce a study based on a published paper and come up against issues, it is notoriously difficult to replicate science. This can result from, missing details in the method, variation in equipment, or operator error. One of the most prevalent reasons, and the reason we will explore in this article today, is that the cell lines are not what you they think they are.
The rates of reproducing experiments vary across disciplines; however, research has shown that 70% of scientists have experienced this problem, and 50% have even failed to reproduce their own work. [1] Reproducibility rates for cancer biology are thought to be as low as 10%, implying that 90% of studies cannot be repeated.
A significant impact of in vitro studies not being reproducible is that unreliable data can lead to unnecessary or misaligned clinical studies which are large and costly. Due to the reproducibility crisis, participants are potentially being subjected to testing that may turn out to be unsuccessful, caused by pre-clinical results that are later found not be reproducible.
So why is it difficult to replicate published papers?
In cell culture, cells are observed using microscopy, however the cells may not be what you think they are. Bacterial and Fungal contamination is often clearly visible using microscopy, and usually can be detected without microscopy via a change in the media. However, mycoplasma and viruses are too small to be visualised under microscopy (even with DAPI stain and a fluorescence microscope the results are considered unreliable)
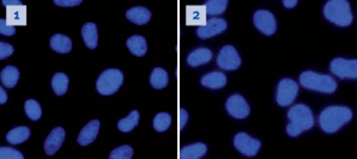
Image 1 shows a Mycoplasma negative culture and Image 2 shows a Mycoplasma positive culture.
Mycoplasma are among the smallest bacteria, capable of passing through filters with a pore size of 0.2 microns. They do not usually kill cells but can impact cultures by altering cellar metabolism, chromosomal aberrations, slowing cell growth and interfering with cell attachment. All these factors can cause the cells to react differently in test to non-infected cells. Mycoplasma can spread quickly between flasks and can infect different cell lines in the same lab.
Mis-identified or cross-contaminated cell lines may be identified by experienced operators via subtle differences in cell morphology, but these differences can be easy to miss.
How to have confidence in your cell lines
It is not all doom and gloom! Misidentified and contaminated cell lines can be identified by cell line authentication. Mycoplasma and viral contamination can be identified by qPCR. At Perfectus Biomed Group we test our cell lines on a regular basis to ensure the data we generate for our clients is of the highest quality.
Typically, if you receive a vial from a trusted source, this vial is then expanded in culture to create the master cell bank. Vials will be expanded at various intervals to create a working cell bank. In order to maintain confidence in your cell line, we recommend testing at three key points; 1) the master cell bank, 2) the working cell bank and 3) throughout ongoing testing. Regularly assessing testing your master cell bank is crucial as it could be in use for over a decade and could be used for millions of pounds worth of research. Regular cell line assessments enhance your confidence in your work but they are also beneficial in obtaining grant funding. Including a plan to screen cells adds weight to grant applications, and at publication, where more and more high ranking journal are demanding evidence of appropriate cell line assessments.
Perfectus Biomed Group can support your research with our cell line quality check program. The programme is carried out in line with ATCC ASN-0002-2011 standard (for the identification of cases of misidentification and cross contamination of cell lines). Our turnaround times can be as little as 48 hours, and project reports are simple, easy to follow and can be submitted to journals with a paper.
If you would like more information on our cell line authentication services please contact us today.
[1] M, Baker & D, Penny Nature Vol: 533, 452 – 454 (2016).

