BS EN 1276
VIRAL TESTING SPECIALISTS
BS EN 1276: 2019 – Quantitative suspension test for the evaluation of bactericidal activity of chemical disinfectants and antiseptics used in food, industrial, domestic and institutional areas
Test Conditions
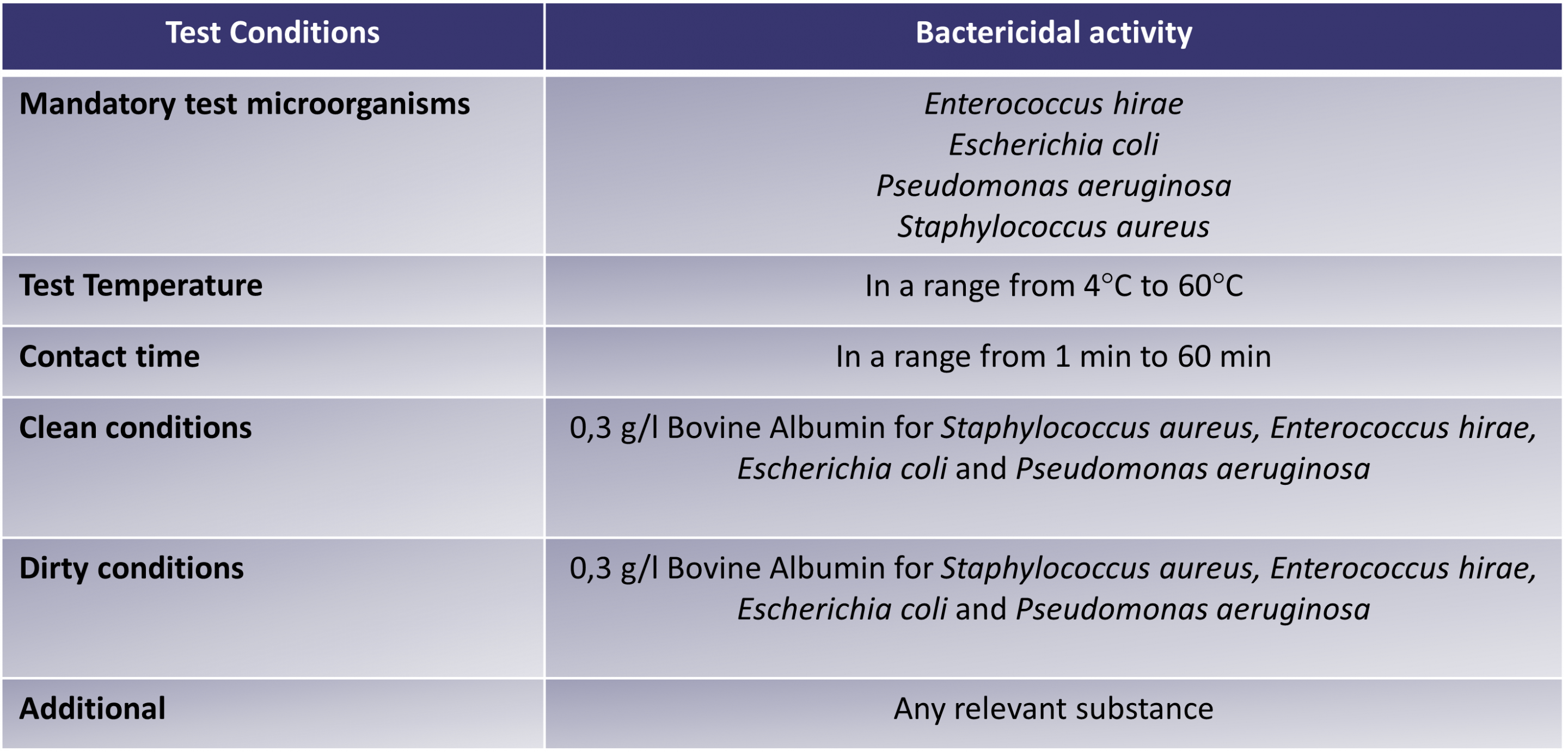
The below table outlines the minimum requirements for the EN 1276 test – Table 1: test conditions for general purpose disinfection:
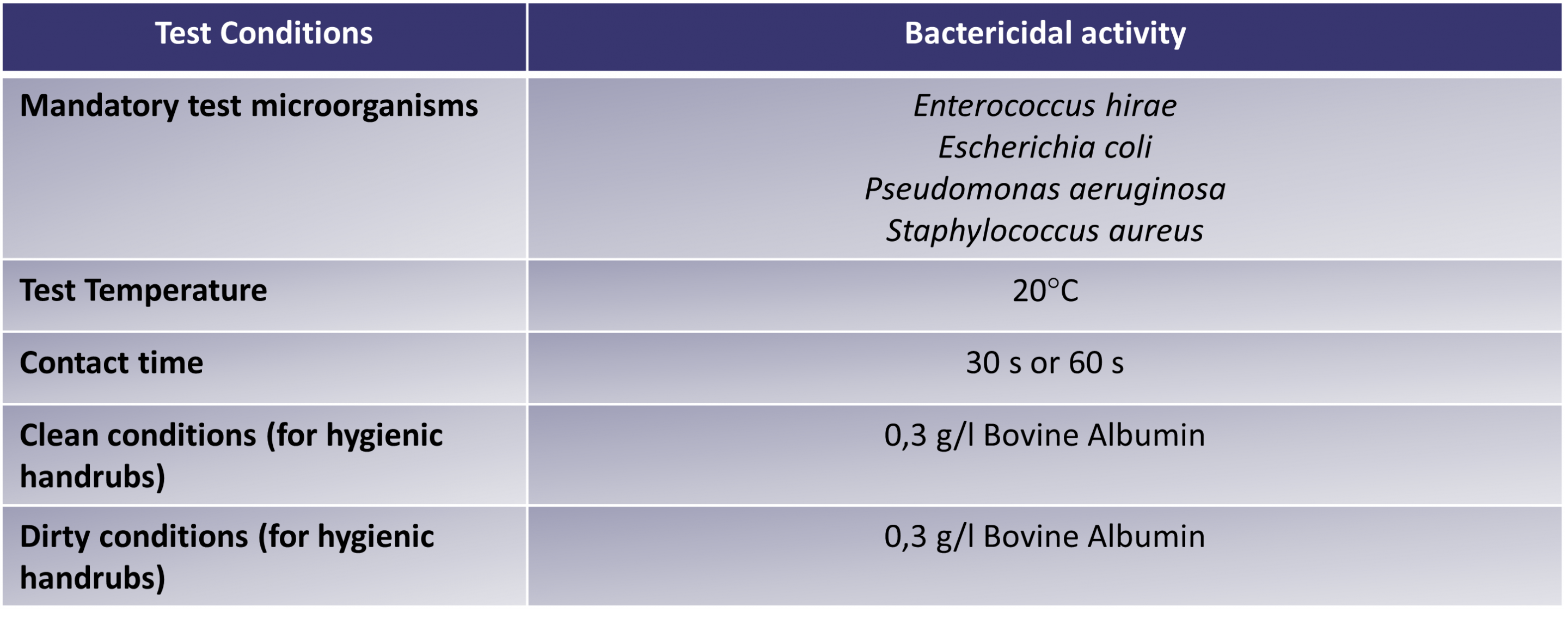
Table 2: test conditions for hand hygiene:
This standard describes a suspension test for establishing whether a chemical disinfectant or antiseptic has or does not have bactericidal activity described in the scope.
Common industries that require this standard for a product
- the processing, distribution and retailing of:
- milk & milk products
- meat & meat products
- fish, seafood & related products
- eggs & egg products
- animal feeds
- other industry areas:
- catering
- textiles
- public transport
- schools
- sports rooms
- pharmaceutical
- cosmetics and toiletries
A sample of the product is added to a test suspension of viruses in a solution of an interfering substance. The mixture is allowed to interact for the duration of the contact time. The final mixture is then acquired and incubated for 2 days to allow surviving bacteria (if any) to proliferate. The bacterial colony is counted and compared against the original culture size.