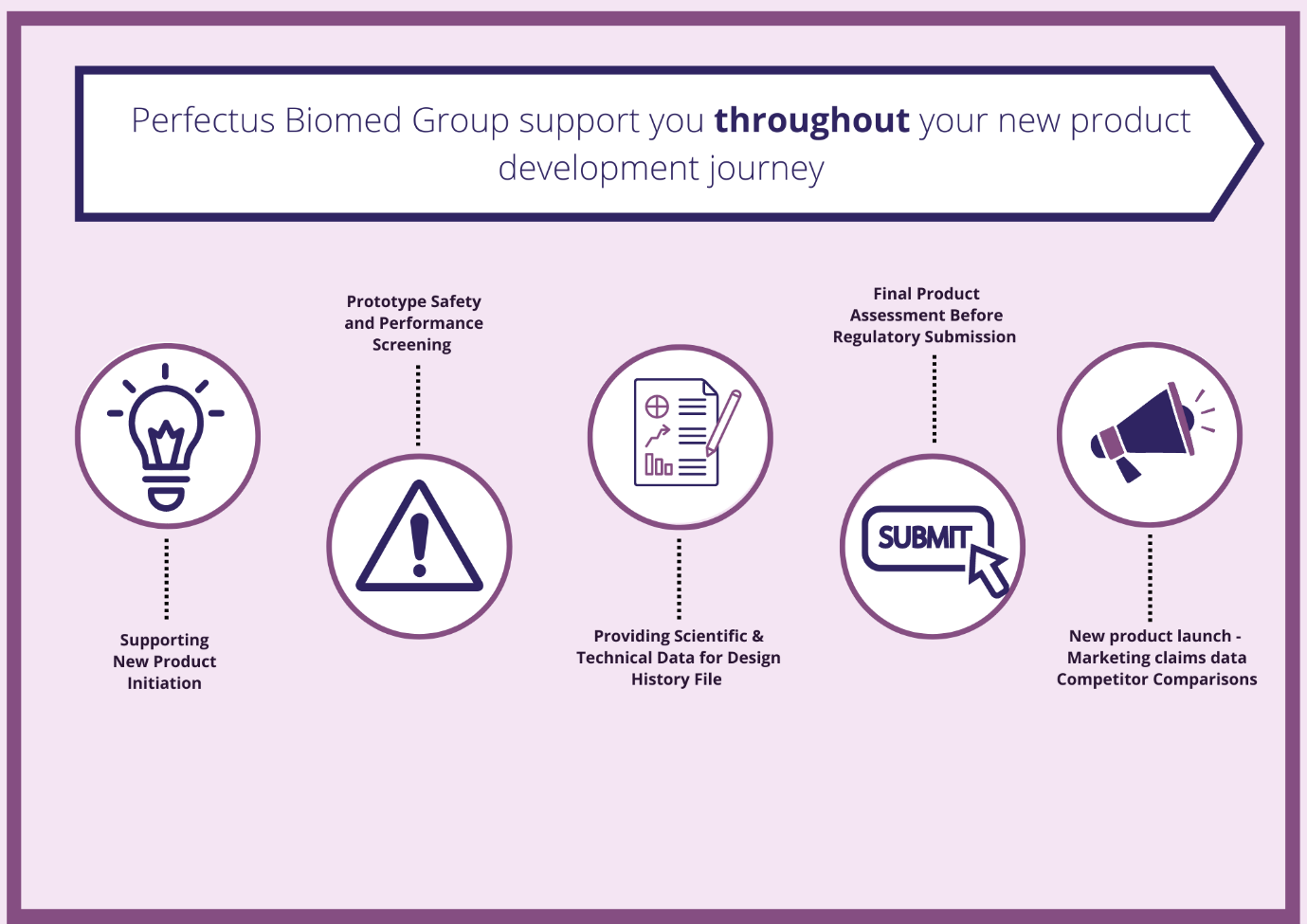
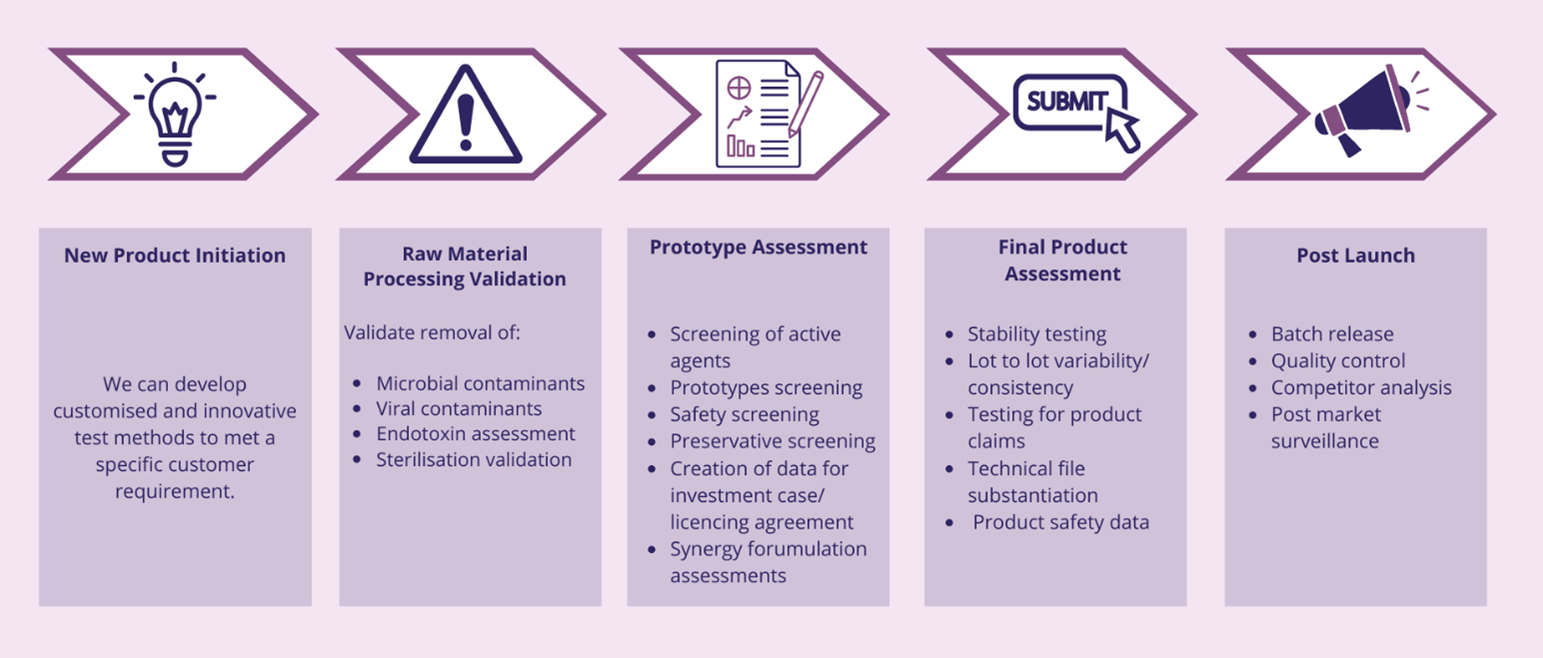
As the regulatory requirements of bringing a product to market continue to increase, the product development journey requires extensive experience in standard and customised microbiological and biological solutions . To bring forward a product that ultimately improves patient’s lives, it is critical to understand the stages and complexities of new product development, and the nuanced requirements of different regulatory bodies.
Regulatory Support and Navigation
Using standardised & validated customised in vitro & ex vivo models we can generate data to support your product development journey. Examples include:
- Screening prototypes for safety and performance (choosing a lead prototype).
- Design verification testing data to prove that the product was designed correctly).
- Design validation (data to prove that the product meets customer requirements).
- ISO 10993 biocompatibility testing (data to demonstrate the product is safe).
- Generating data to support regulatory and commercial claims.
- Providing data for your product Design History File.
Below are some of the test methods you may require at different stages of the medical device product development process :
EU Medical Device Regulation (MDR)
Regulatory bodies, approving medical device submissions, have become more stringent and in-depth in recent years. In 2021 the EU Medical Device Regulations (MDR) replaced the Medical Device Directive (MDD). There is an increased requirement for companies to rationalize their portfolios and perform a global impact assessment, Unique Device Identifications (UDI) have been rolled out across different classes of medical device and a greater focus on safety is demanded (the word safety appears 290 more times in the MDR compared to the MDD!1)
A crucial element in the product development process for medical devices is to ensure that safety and performance/ efficacy requirements satisfy regulatory submissions.
Our customised and specialist scientific services are built to meet our clients needs. We have extensive experience in supporting clients bring products to market. We look forward to hearing about any current or planned projects you have. Contact us to arrange a call.
References:
1) https://www.thefdagroup.com/blog/mdr-vs-mdd-13-key-changes