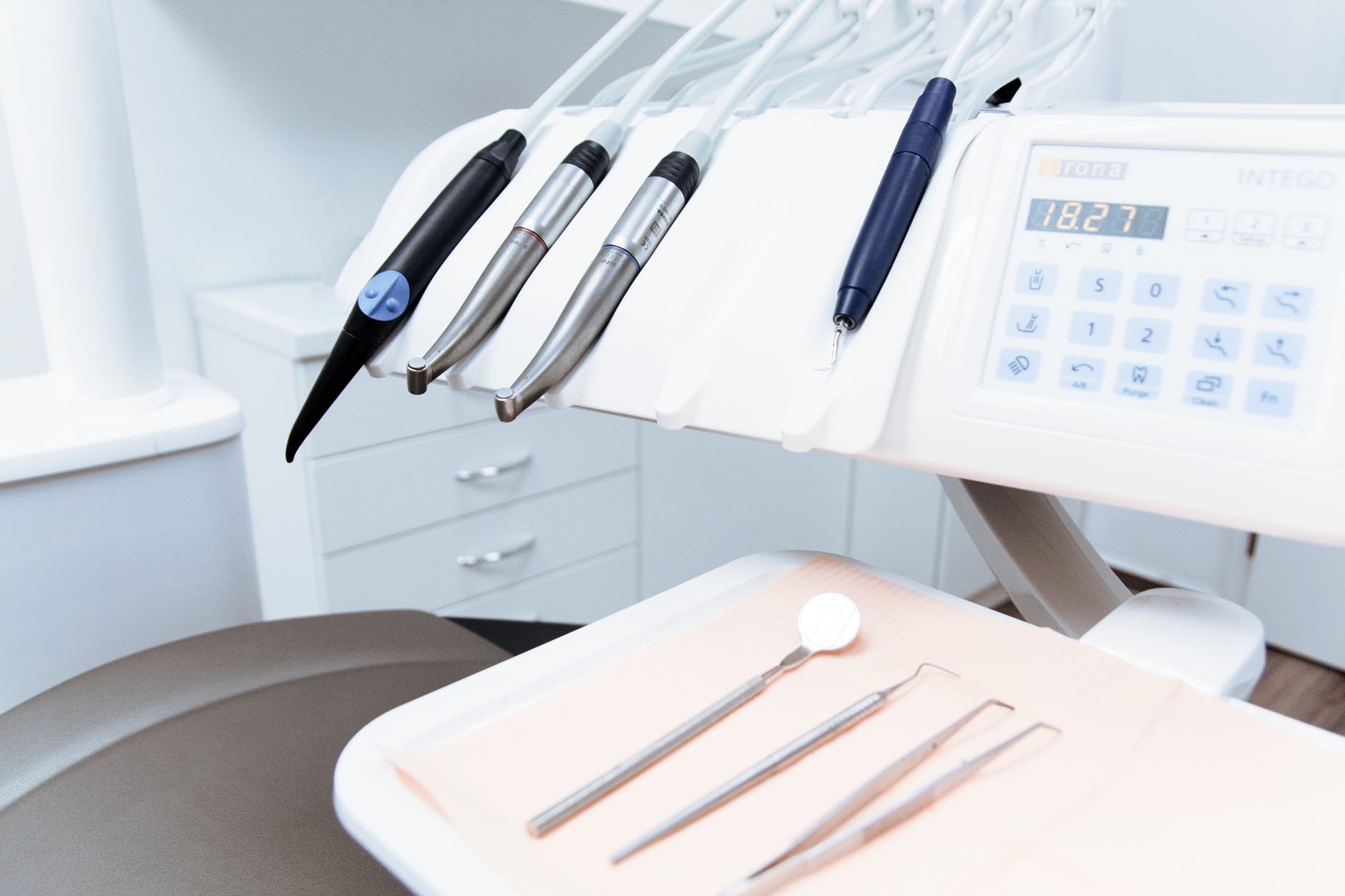
The U.S. Food and Drug Administration (FDA) have recently published guidelines on the importance of infection control in Dental Unit Waterlines (DUWL). The guidelines also highlight the ‘do’s and don’ts’ for dentists.
Microbial contamination of DUWL can arise in a number of ways, from the mains water supply to the oral bacteria from the dental hand piece. Three of the main problematic bacteria found in DUWL are Legionella spp., Mycobacterium spp. and Pseudomonas species. The waterlines in a dental unit are constructed from a polymer or silicone rubber tubing. They provide water for irrigation, cooling and flushing of oral cavities. Dental units are therefore regulated by the FDA and require 510(k) premarket clearance.
Infection Control in Dental Unit Waterlines
Dental unit waterlines can use water from municipal water sources or closed-bottle systems. While water from municipal sources is considered safe to drink, DUWL cannot be sterilised and so the bacteria found within this water could pose an infection risk to patients and dental professionals. Without the proper cleaning and disinfection, waterborne microorganisms can form a biofilm within the waterlines. The biofilms can become dislodged and enter the water stream. Below are the main contributing factors to biofilm formation within DUWL:
- Overnight stagnation
- The large surface area to volume ratio of DUWL tubing
- Low levels of water use
Current guidelines for treating the water within DUWL include treatment with commercial products to meet drinking water standards, recommended by the Centres for Disease Control and Prevention (CDC), as well as routine water quality monitoring to demonstrate bacteria count of less than or equal to 500 Colony Forming Units (CFU) per millilitre of heterotrophic bacteria, suggested by the American Dental Association (ADA).
FDA Guidelines
- Establish written standard operating procedures to outline infection control in dental unit waterlines
- Implement equipment to aid water quality, such as filtration systems and chemical treatment protocols
- After each patient, clear the water and air lines for a minimum or 20-30 seconds to flush out any patient material that may have entered the waterline
- Take note of signs indicative of biofilm formation, such as musty odours, cloudiness within the water and clogging of the lines.
For a full list of the infection control tips from the FDA and reprocessing instructions for DUWL, please visit the FDA website.
Perfectus Biomed offer a range of dental biofilm test methods and can also support your 510(k) submission. If you would like to discuss this in more detail, please contact us.