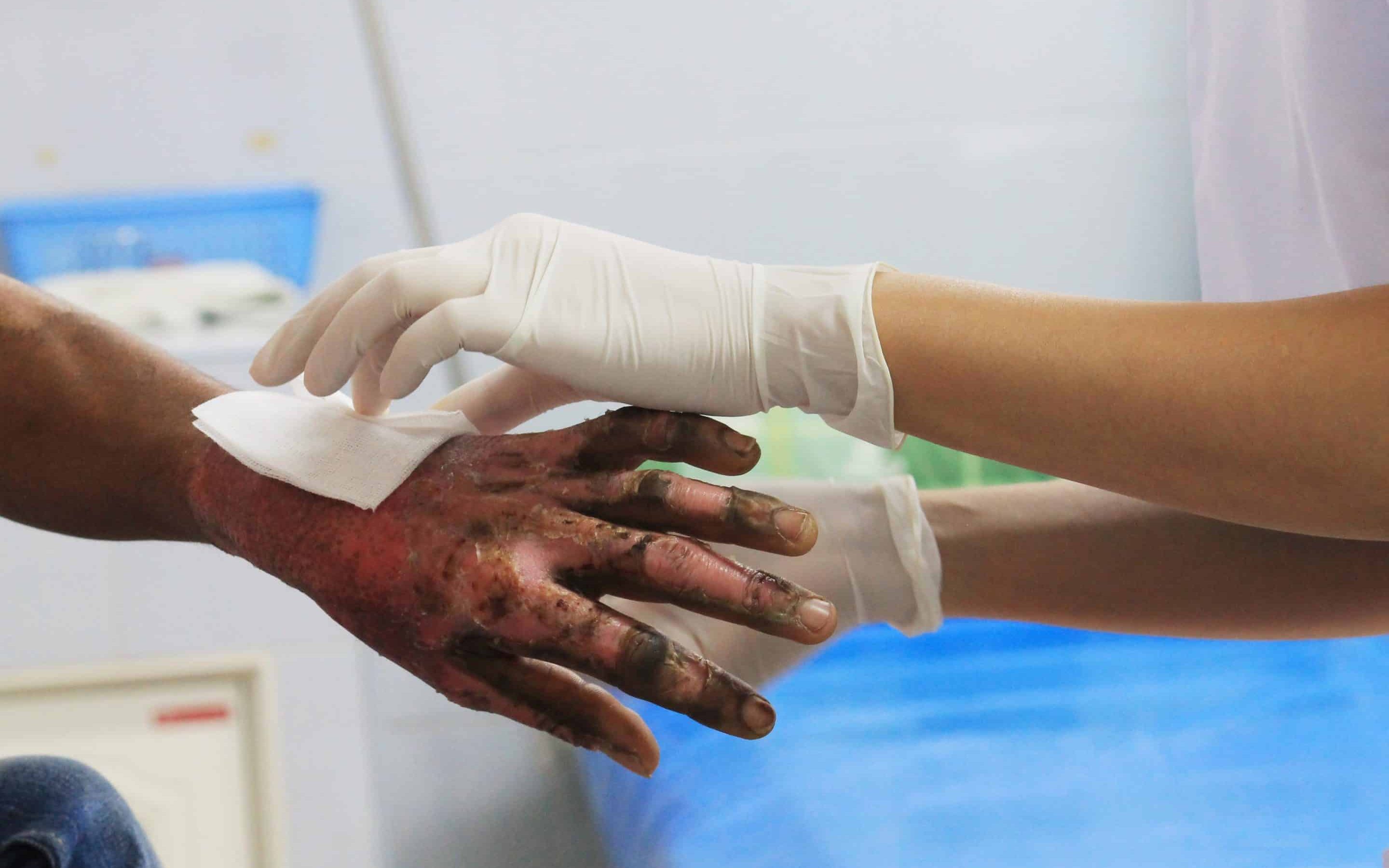
We are thrilled to announce that we have recently received UKAS ISO 17025 accreditation for our ex vivo tissue Burn Wound Model. This latest accreditation adds to our impressive list of accredited biofilm methods, both in vitro and ex vivo. Well done to our fantastic, hard working and innovative team for adding yet another method to our UKAS schedule of accreditation.
Biofilms and burn wounds
Burns are one of the most common and devastating forms of trauma, the National Centre for Injury Prevention and Control in the United States reports that approximately 2 million fires are reported each year which result in 1.2 million people with burn injuries.1 It is estimated that more than 70% of deaths occur in burn patients due to infection.2 When bacteria within infected wounds exist as biofilms this leads to additional treatment challenges in wound management . Biofilms in burn wounds are a major clinical problem, and 60% of burn mortality is attributed to biofilms.3 Bacterial communities including biofilms can also develop antibiotic resistance, further complicating treatment. Topical dressings and antibiotics are applied to burn wounds to inhibit bacterial growth and replication. To assess the ability of an antimicrobial agent to prevent biofilm formation or breakdown an established biofilm, it is first important to be able to grow reproducible, clinically relevant biofilms. The ex vivo burn wound model is used to grow reproducible biofilms on the surface of porcine skin under defined conditions which are suitable for efficacy testing.
What is the ex vivo Burn Wound Model?
It has been reported that there are 74% in vivo, 23% in vitro and 3% ex vivo models available to test therapies for burn wound infections.4 In vivo studies have significantly enhanced the understanding of wound biofilm formation. Basic in vitro models do not fully mimic the complexities of a clinical burn wound however, the ex vivo porcine skin provides the best representation of human skin and wound healing, without the ethical and financial factors that come with animal testing. The robust, reproducible data can be used to support clinical trials.
The ex vivo burn wound model test method is adapted by Perfectus Biomed from a methodology published by the University of Bath. The method involves the growth of repeatable Pseudomonas aeruginosa and Staphylococcus aureus biofilms on individual burn wounds on the surface of ex vivo porcine skin. This model replicates a generalised situation where biofilm exists within burn wounds of patients. The importance of this model being ex vivo should not be underestimated as it allows for the development of bacterial communities more similar to those in a clinical burn wound.
Type of products that can use this test method
The test method can be used to enable the pre-clinical efficacy of antimicrobial agents such as antibiotics and dressings. The choice of organisms can be customised dependant on the requirements. Pseudomonas aeruginosa and methicillin-resistant Staphylococcus aureus are key pathogens in burn wound infections. The model can also be used to differentiate product efficacy both within and between product groups. This is a critical tool in helping to screen appropriate formulations and giving healthcare practitioners the confidence in products that are likely to be effective within clinical settings.
The benefits of ISO 17025 accredited test methods
ISO 17025 accreditation is an internationally recognised standard and methods accredited to this standard ensure confidence by underpinning quality of results and ensuring traceability, comparability, and validity. The validation of this model represents the first burn wound model to be accredited by UKAS to ISO 17025.
This latest accreditation adds to our schedule of accreditation which includes the Single Tube Method, Drip Flow Biofilm Reactor, CDC Biofilm Reactor, Minimum Biofilm Eradication Concentration (MBEC), and ex vivo porcine lung model which can be used to test topical and aerosolised treatments for infected mucosal wounds.
For more information on any of these models or to find out how our biofilm testing expertise can support your project, contact us today.
References
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1471990/
- https://applications.emro.who.int/imemrf/Professional_Med_J_Q/Professional_Med_J_Q_2014_21_5_869_873.pdf
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8702030/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8702030/