Biofilm Awareness Week
Perfectus Biomed Group are excited to support The National Biofilms Innovation Centre (NBIC) #BiofilmWeek, highlighting the research taking place to prevent, detect, manage, and engineer biofilms. Perfectus Biomed’s mission is to harness innovative science to improve lives, we do this by developing ‘fit for purpose’ experiments that mimic ‘real-life’ scenarios. Our ISO 17025 accredited Porcine Lung Model is key in helping companies to screen and test Cystic Fibrosis treatment candidates.
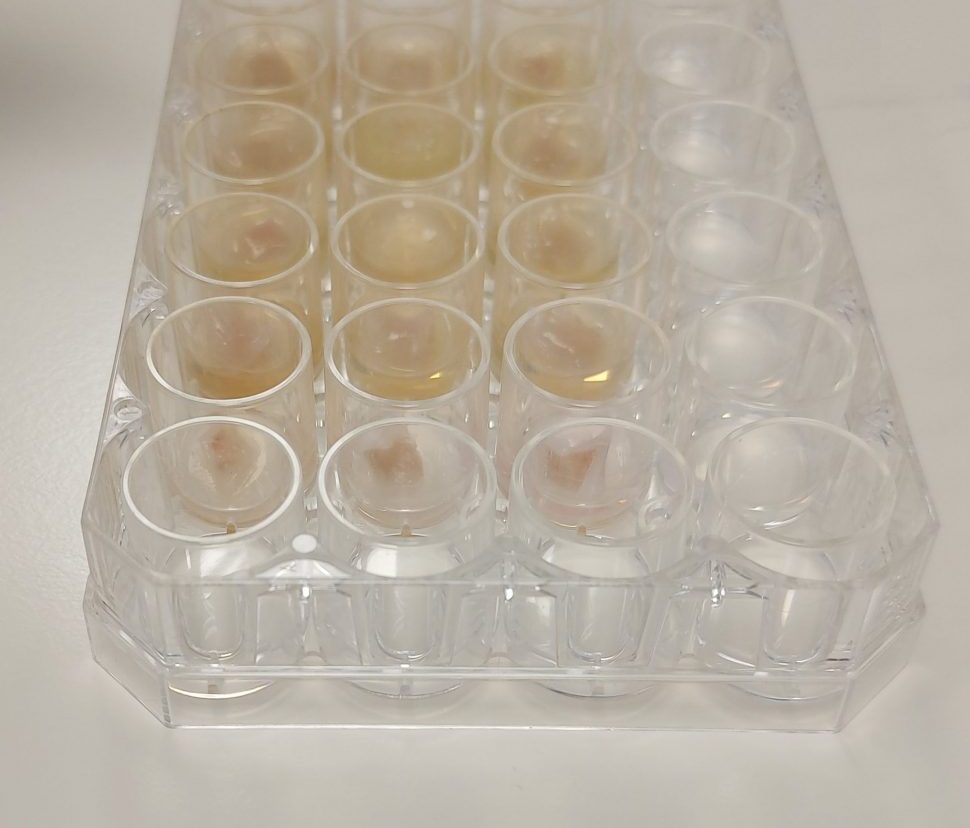
Perfectus Biomed worked with the University of Warwick to understand the models’ capabilities and then received UKAS validation for the model in 2014. The model uses porcine lung (from animals destined for human consumption) as a mimic of human lung tissue. This allows us to study the biofilm growth of bacteria like Pseudomonas aeruginosa and understand the efficacy of antimicrobial products such as pharmaceuticals against these biofilms. Patients with diseases such as cystic fibrosis are more prone to lung infections from this organism. The model has been developed so that it replicates these infections as closely as possible in an ex vivo manner, using the mucosal epithelial layer of the bronchiole, and artificial sputum medium to simulate the sticky mucus produced in the lungs.
This method can also be used to test topical and aerosolised treatments for infected mucosal wounds in line with ISO 17025 regulations. Mucosal tissues such as those present in the gastrointestinal tract, nasal passages and urinary tract are vulnerable to pressures which can cause them to become ulcerated. These pressures can include medical devices, such as oxygen tubing, endotracheal tubes and urinary catheters, among others.
Perfectus Biomed Group are market leading in accredited biofilm methods, and currently no other laboratory offers a testing service to ISO 17025 for this test method or similar.
This model can be used for newly developed pharmaceuticals and other antimicrobial agents prior to clinical trials, which have the potential to improve the quality of life for patients suffering from repeated lung infections as a result of P. aeruginosa colonizing the sticky mucus produced by cystic fibrosis patients.
For more information on our accredited and customisable biofilm methods please contact us.