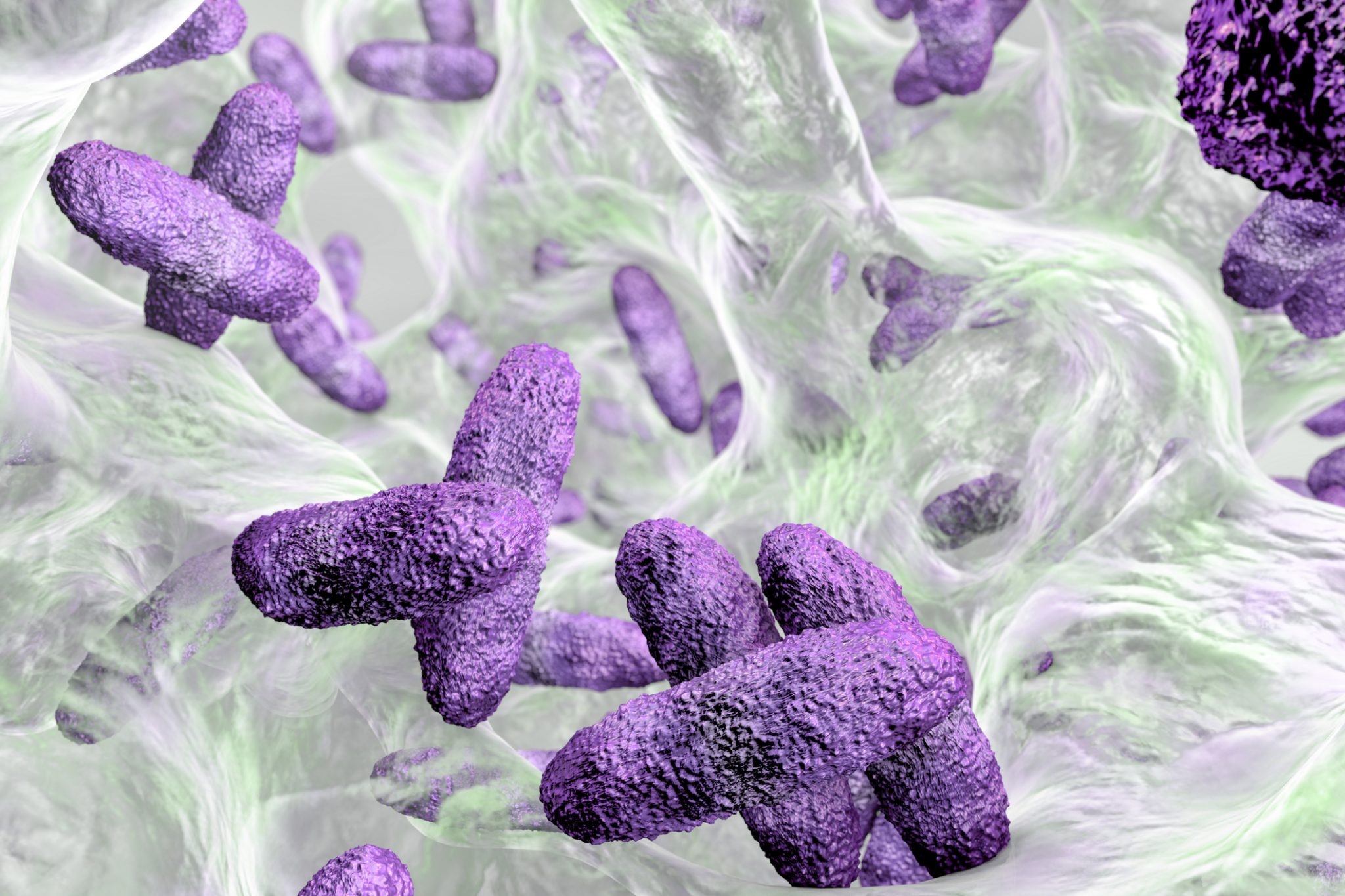
Congratulations to Steris Corporation on achieving a biofilm removal label claim for the STERIS ProKlenz® ONE Alkaline cleaner.
Biofilm removal claims demonstrate a products’ ability to remove biofilms, one of the biggest antimicrobial challenges across many sectors. Microbial contamination, if not eradicated, can lead to the formation of biofilms. Within a bacterial biofilm, microorganisms are encased within a self-produced Extracellular Polymeric Substance (EPS) that decreases susceptibility to antimicrobial agents and environmental stresses. The ability of a product to remove biofilm from a surface is critical to the development of products that effectively remove contamination in the ‘real world’.
How to obtain a biofilm removal label claim
Biofilm removal claims are achieved through extensive testing of the removal of biofilms from hard, non-porous surfaces. In order to register an antimicrobial product for a public health biofilm removal claim, the Environmental Protection Agency (EPA) require efficacy data to be submitted under the Federal Insecticide, Fungicide and Rodenticide Act (FIFRA). The EPA have two complementary methods that are required to evaluate the efficacy of antimicrobial pesticides against two biofilm forming genus, Pseudomonas aeruginosa and Staphylococcus aureus. The two methods are outlined below:
- MB-19: Growing a Biofilm using the CDC Biofilm Reactor. This method describes how to develop reproducible biofilms on coupons within a turbulent flow reactor.
- MB-20: Single Tube Method for Determining the Efficacy of Disinfectants against Bacterial Biofilms. This method describes how to measure the efficacy of biocides against biofilm encased bacteria.

Contact us to understand how to perform these methods in line with EPA requirements in order to achieve a successful biofilm label claim.

 Tissue Models
Tissue Models Biocompatibility & Safety
Biocompatibility & Safety Viral Testing
Viral Testing Molecular Testing
Molecular Testing Biofilm Testing
Biofilm Testing Microbiology Testing & Research
Microbiology Testing & Research Cell Banking
Cell Banking Medical Devices
Medical Devices Wound Care
Wound Care Biocides & Disinfectants
Biocides & Disinfectants Infectious Diseases
Infectious Diseases Personal Care & Cosmetic Testing
Personal Care & Cosmetic Testing Industrial Biofilms
Industrial Biofilms Orthopaedic
Orthopaedic Oral Care & Dentistry
Oral Care & Dentistry