LATEST NEWS
Cell line quality assurance at every stage of cell banking
Why consider cell banking services?
A proficient researcher understands the problems associated with maintaining a cell line in continuous or extended cell culture. We understand our clients need to ensure cell and data integrity is maintained throughout a controlled...
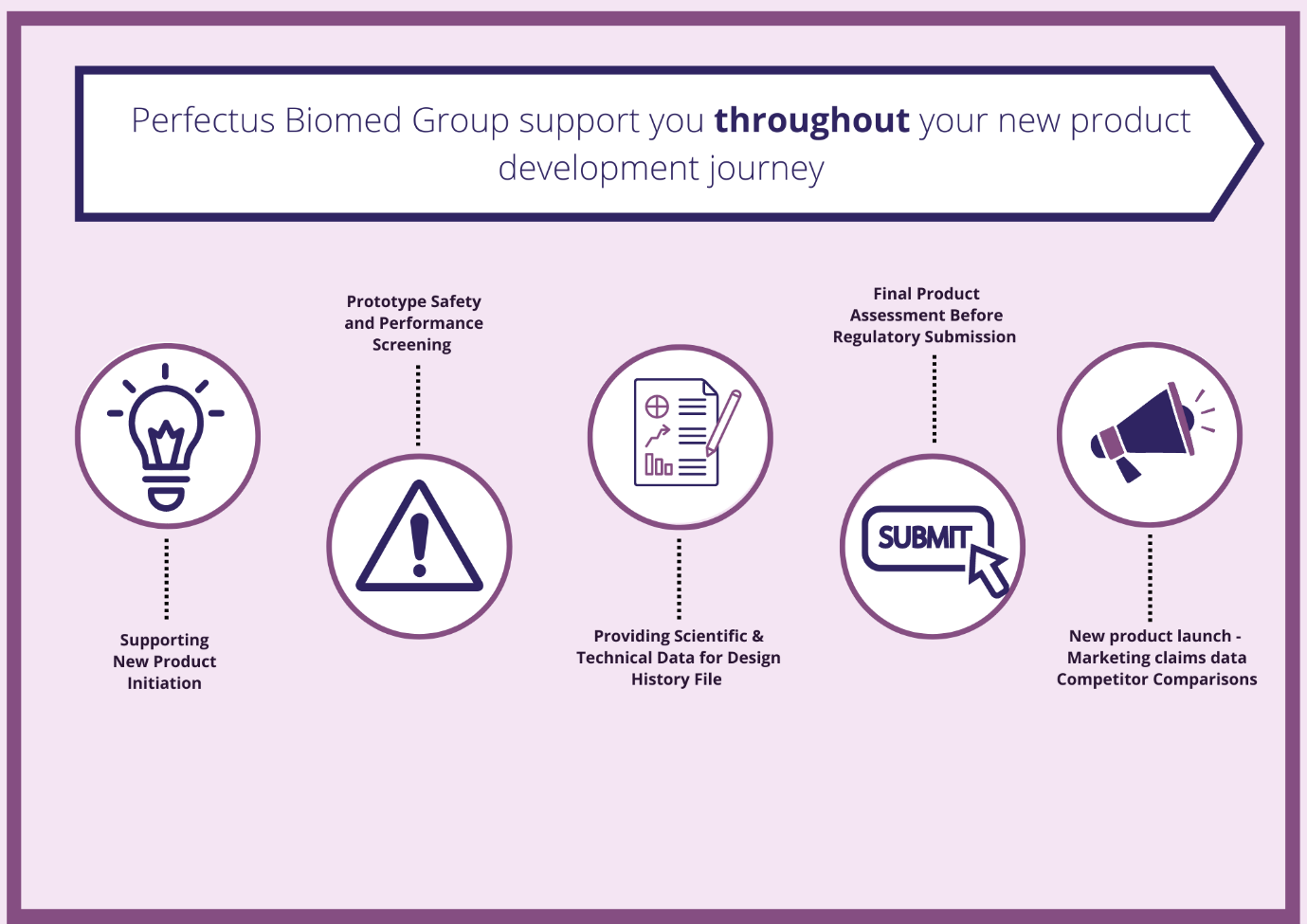
Supporting you on your medical device product development journey
As the regulatory requirements of bringing a product to market continue to increase, the product development journey requires extensive experience in standard and customised microbiological and biological solutions . To bring forward a product that ultimately...

Lateral Flow Devices
The rapid development of the production of lateral flow devices (LFD) over the last two years has been transformative. In the wake of the 2020 global pandemic, regulatory bodies made rapid changes to the requirements for bringing a lateral flow device to market. In...

EN 12791, EN 1500 and EN 1499 – Regulatory Submission for Hand Disinfection Returns
H]Throughout the pandemic the burden of data required to take a hand wash product to market was significantly reduced. As we emerge from the last two years regulatory requirements are changing to again include safety testing, efficacy testing and the inclusion of a...
AMR and Biofilm – A Relationship We Must Acknowledge in Our Efforts to Fight AMR
AMR and Biofilm – What Does It Mean?
Antimicrobial resistance (AMR) is a global health threat referred to as the ‘silent pandemic’. The World Health Organisation (WHO) has declared that AMR is one of the top 10 global public health threats facing humanity,1 and it has...
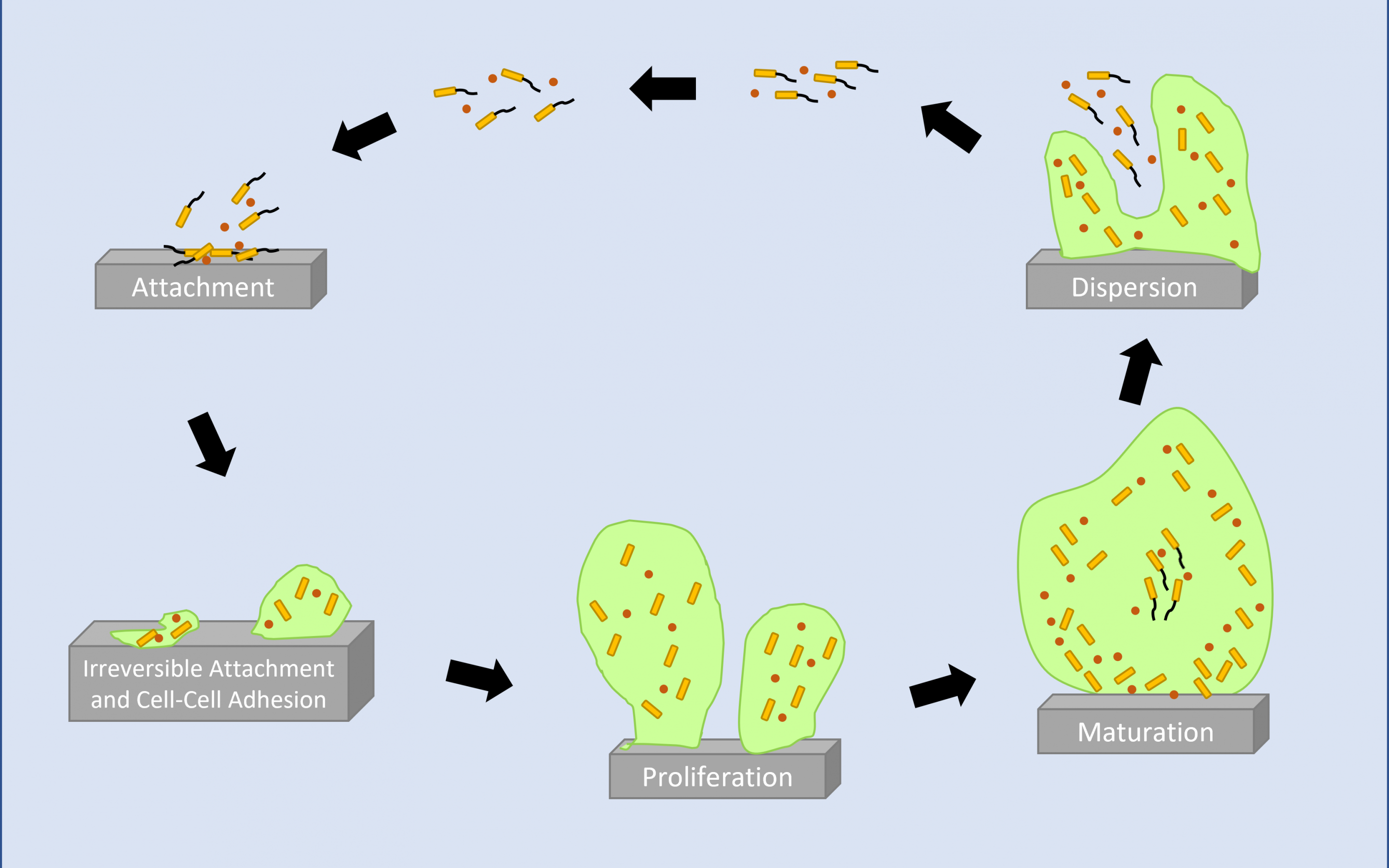
Biofilms – A Key World Issue
Back to Basics - What is a Biofilm and How is it Formed?
In the simplest terms, biofilms are formed when microorganisms, such as bacteria, yeast and fungi attach and proliferate on a surface. Once attached the microorganisms form and reside within a self-produced...

Perfectus Biomed Announces New Laboratory Expansion
The Perfectus Biomed team is thrilled to announce that we have once again expanded our UK laboratory space, and it is now up and running!
The Benefits of Our New Space
The newest facilities expansion allows us to further support our clients and continue to deliver innovative...

Congratulations to Laura Sellers, who has graduated from the Morgan James Strategic Leaders course
Congratulations to our Head of Molecular and Microbiology, Laura Sellers, on her graduation from the Morgan James Strategic Leaders course. The course ran over nine modules and supports its candidates to develop strategic leadership skills while also improving their ability...

A look-back on 2021 at Perfectus Biomed Group
From the whole team at Perfectus Biomed Group, we wish our valued clients, associates, and collaborators a very Merry Christmas and a Happy New Year.
As we approach the end of 2021 we wanted to take the time to look back on the last year at Perfectus Biomed Group, and some...
